Part 1/6: The Antibiotic Battlefield: How Antibiotics Affect Your Microbiome
Introduction to Antibiotics and Their Mechanisms; The Prevalence of Antibiotic Prescription
Previous
Welcome GutSphere Friends,
Welcome to Part 1: The Antibiotic Battlefield: How Antibiotics Affect our Microbiome. Together, we're about to delve into an exciting journey, diving deep into the heart of a fascinating story that began almost a century ago.
In the 1920s, a serendipitous discovery by Sir Alexander Fleming opened a new era in medicine - the era of antibiotics[1]. Since then, these remarkable drugs have stood as bulwarks against bacterial infections, saving millions of lives and changing the trajectory of public health. In fact, according to the World Health Organization, an estimated millions of lives have been saved by antibiotics in the last century alone[2].
As we stand on this side of history, the magnitude of antibiotics' contribution to our health is undeniable. But like all great stories, there's another side that's often overlooked. That's the story we aim to unravel together – the impact of antibiotics on our gut microbiome, an intricate community of microorganisms that reside within us.
In this first chapter, we will lay down the groundwork, demystifying the nature and mechanisms of antibiotics. We'll explore the different types of antibiotics, each with their unique strategies to vanquish bacterial invaders, enriching our understanding of this invisible battlefield.
Moving forward, we'll investigate the prevalence of antibiotic prescriptions. It's a startling fact that, according to data from the Center for Disease Dynamics, Economics & Policy, global antibiotic use has risen by 65% from 2000 to 2015[3]. The patterns of prescription and consumption, however, vary widely between countries, presenting a complex picture of global antibiotic usage.
Throughout our journey, we'll ground our discussions on credible, evidence-based insights. So buckle up as we pull back the curtain and embark on an enlightening exploration of antibiotics and the microbiome. Welcome to the battlefield!
References:
The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5403050/
Antimicrobial resistance: conserving life-saving medicines takes everyone’s help, https://www.who.int/news-room/feature-stories/detail/antimicrobial-resistance-conserving-life-saving-medicines-takes-everyone-s-help
Global increase and geographic convergence in antibiotic consumption between 2000 and 2015, https://www.pnas.org/doi/10.1073/pnas.1717295115
Battle Inside: Antibiotics vs Your Gut
Introduction to Antibiotics and Their Mechanisms
A crucial first step to lay the groundwork for the rest of the discussion. Explaining different types of antibiotics and how they work against bacteria.
As we step into the invisible battlefield that unfolds inside us each time we take an antibiotic, it's vital that we begin by understanding the soldiers in this war: the antibiotics themselves. These miraculous medical interventions have been with us for less than a century, but in that time, they've profoundly reshaped the face of human health.
Antibiotics, by definition, are substances or compounds that can kill or inhibit the growth of bacteria. Despite their name, which translates to "against life," they don't target all life—just bacterial life. Our bodies are home to an array of beneficial bacteria, particularly in our gut, but there are also harmful bacteria that can cause serious diseases. When these harmful bacteria invade and proliferate, antibiotics can be life-saving allies.
The key to the power of antibiotics lies in their selective toxicity. They exploit the biological differences between human cells and bacterial cells. For example, human cells don't have cell walls, but many bacteria do. Antibiotics like penicillin target the process of cell wall synthesis, causing bacteria to burst open and die while leaving human cells unharmed.
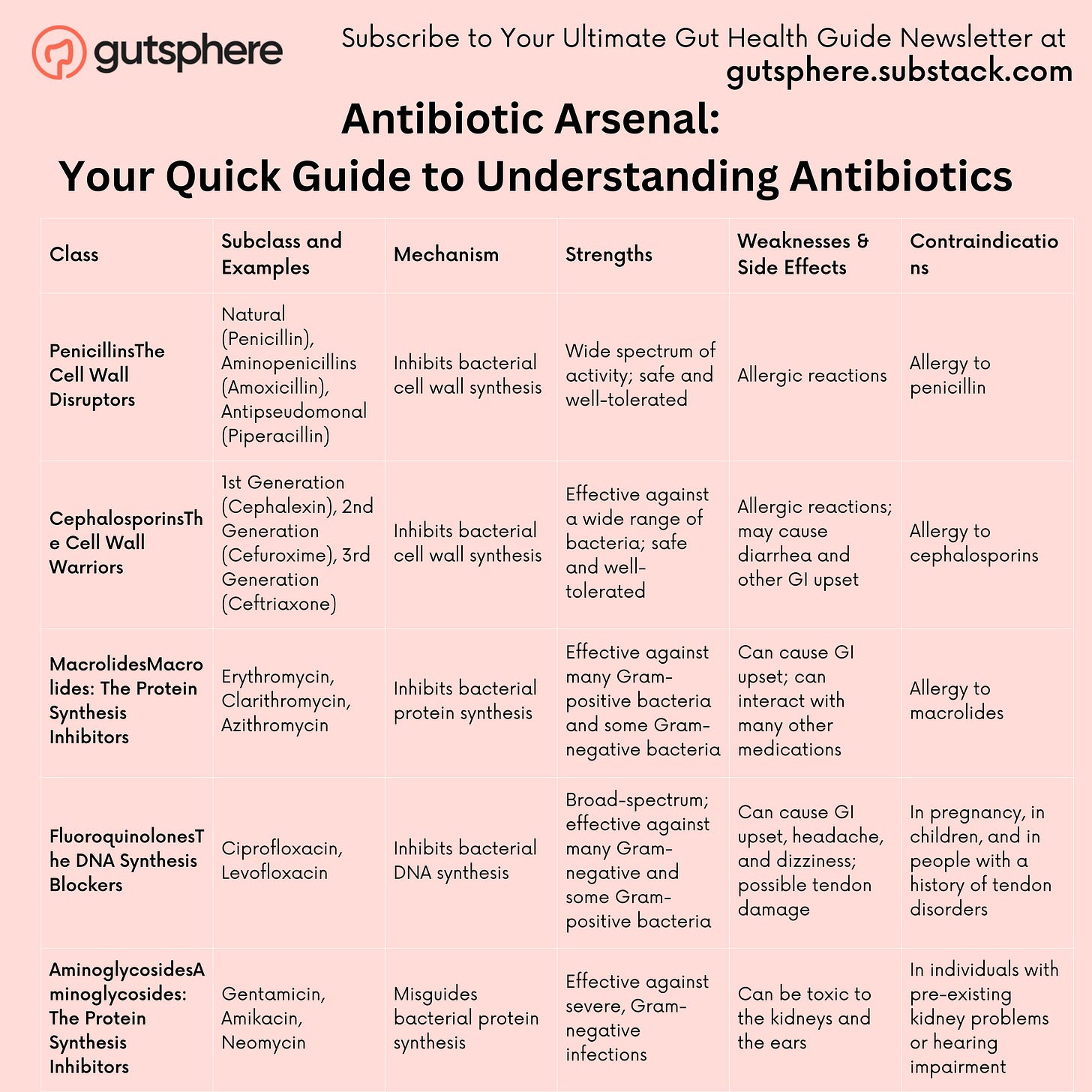
Antibiotics are not a monolithic group. They come in many forms, each with a unique mode of action. There are five main classes: penicillins, cephalosporins, macrolides, fluoroquinolones, and aminoglycosides.
Penicillins:The Cell Wall Disruptors
Cephalosporins: The Cell Wall Warriors
Macrolides: The Protein Synthesis Inhibitors
Fluoroquinolones: The DNA Synthesis Blockers
Aminoglycosides: The Protein Synthesis Inhibitors
Before we elaborate in detail. The table below summarizes the antibiotics and their mechanisms.
Antibiotic Arsenal: Your Quick Guide to Understanding Antibiotics
1. Penicillins: The Cell Wall Disruptors
Subclasses and Examples: Penicillins can be further divided into natural penicillins (like Penicillin G and V), penicillinase-resistant penicillins (Methicillin, Oxacillin), aminopenicillins (Amoxicillin, Ampicillin), and antipseudomonal penicillins (Piperacillin, Ticarcillin).
How They Work: As we mentioned earlier, penicillins are like demolition experts, targeting the cell walls of bacteria. They disrupt the process of cell wall synthesis, which causes the bacteria to quite literally burst from osmotic pressure.
Strengths: This ability to break down the cell wall is quite effective against a wide range of Gram-positive bacteria, and some Gram-negative bacteria too.
Weaknesses & Side Effects: Some bacteria have evolved to produce enzymes called beta-lactamases that can break down penicillin, making it ineffective. These resistant strains are a growing concern. Common side effects of penicillins can include diarrhea, nausea, and skin rash. More serious, but less common side effects include allergic reactions which can be life-threatening.
Contraindications: Those who are allergic to penicillin or have had a previous allergic reaction to a penicillin-class antibiotic should avoid this class of antibiotics.
2. Cephalosporins: The Cell Wall Warriors
Subclasses and Examples: These antibiotics are grouped into "generations" based on their time of discovery and spectrum of antimicrobial activity. The first generation (Cefazolin, Cephalexin), second generation (Cefaclor, Cefuroxime), third generation (Ceftriaxone, Ceftazidime), fourth generation (Cefepime), and fifth generation (Ceftaroline) each have their unique characteristics and bacterial targets.
How They Work: Like the penicillins, cephalosporins are excellent at sabotaging bacterial cell walls. They inhibit enzymes responsible for building cell walls, causing the bacteria to become unstable and eventually perish.
Strengths: With each new generation, cephalosporins have evolved to be more effective against a wider range of bacteria. They are broad-spectrum antibiotics, which means they can tackle both Gram-positive and Gram-negative bacteria. The later generations, especially, have better coverage of Gram-negative bacteria.
Weaknesses & Side Effects: Unfortunately, some bacteria have learned to resist cephalosporins, just as they have with penicillins. Also, people allergic to penicillins might also react to cephalosporins. Side effects may include diarrhea, stomach cramps, nausea, and vomiting. As with any drug, severe allergic reactions are possible.
Contraindications: Like penicillins, those with known allergies to this class of antibiotics should avoid cephalosporins.
3. Macrolides: The Protein Synthesis Inhibitors
Subclasses and Examples: Macrolides are grouped based on their chemical structure. The most commonly used are Erythromycin, Clarithromycin, and Azithromycin.
How They Work: These antibiotics cunningly bind to the bacterial ribosomes - the protein factories of bacterial cells. By doing this, they prevent these ribosomes from synthesizing proteins, which are essential for bacterial growth and reproduction.
Strengths: Macrolides are often the antibiotic of choice for people who are allergic to penicillin. They're also broad-spectrum, effective against a variety of bacteria, including the ones that cause common respiratory infections.
Weaknesses & Side Effects: Macrolides can cause stomach upset, and in rare cases, they can lead to severe allergic reactions. There's also an ongoing concern about increasing macrolide resistance, particularly among Streptococcus pneumoniae, a common cause of pneumonia.
Contraindications: People with liver disease or those taking certain other medications (including some cholesterol-lowering drugs) may need to avoid macrolides or use them with caution due to potential drug interactions.
4. Fluoroquinolones: The DNA Synthesis Blockers
Subclasses and Examples: This class includes well-known antibiotics like Ciprofloxacin, Levofloxacin, and Moxifloxacin.
How They Work: Fluoroquinolones are pretty advanced in their tactics. They target bacterial DNA synthesis, thereby blocking their ability to reproduce. More specifically, they inhibit two bacterial enzymes (DNA gyrase and topoisomerase IV) necessary for the bacteria to copy their DNA.
Strengths: The beauty of fluoroquinolones is that they're broad-spectrum and can be effective against both Gram-positive and Gram-negative bacteria. This versatility makes them a valuable tool in treating a wide range of infections.
Weaknesses & Side Effects: These antibiotics are mighty, but their strength comes with side effects. Some common ones include nausea, diarrhea, and dizziness. But, in rare cases, they can cause more serious side effects like tendon damage, heart problems, and even mental health effects. Because of these risks, fluoroquinolones are typically reserved for serious bacterial infections when other antibiotics won't work.
Contraindications: People with certain medical conditions like heart rhythm problems, joint issues, or nervous system conditions should usually avoid fluoroquinolones. They should also be used with caution in elderly populations due to an increased risk of tendon rupture.
5. Aminoglycosides: The Protein Synthesis Inhibitors
Subclasses and Examples: This group includes names such as Gentamicin, Amikacin, and Neomycin.
How They Work: Aminoglycosides have a unique approach. Like Macrolides, they also target protein synthesis, but they do so by binding to a different part of the bacterial ribosome. This misguides the bacteria, leading to the production of faulty proteins, and eventually causing the bacteria's demise.
Strengths: Aminoglycosides are often the heavy hitters when it comes to dealing with severe infections, especially those caused by Gram-negative bacteria. They're also often used in combination with other antibiotics to cover a broader spectrum of bacteria.
Weaknesses & Side Effects: As powerful as aminoglycosides are, they do come with some significant drawbacks. They can be toxic to the kidneys and the ears, causing kidney damage and hearing loss in some cases. This is why their use is typically limited to severe, life-threatening infections where benefits outweigh the potential risks.
Contraindications: Given their potential for kidney and ear toxicity, aminoglycosides are used with caution in individuals with pre-existing kidney problems or hearing impairment.
While these antibiotics have been incredibly successful in treating bacterial infections, they are not without their drawbacks. The human body is a complex ecosystem, and introducing antibiotics can upset that balance. Our understanding of these implications, particularly concerning our gut microbiome, has significantly expanded in recent years.
Now that we have a basic understanding of what antibiotics are and how they work, we're ready to delve deeper into the prevalence of antibiotic prescriptions and what this means for us and the invisible world within us. It's not just about fighting off a bacterial infection; it's about understanding the profound implications these medical marvels have on our overall health. But before we jump into that, let's take a moment to understand the global reach and the sheer scale at which antibiotics are being used today.
References:
The Mechanism of Action of Penicillins, https://pubmed.ncbi.nlm.nih.gov/7372662/
Penicillin's Discovery and Antibiotic Resistance: Lessons for the Future?https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5369031/
Cephalosporins:https://www.ncbi.nlm.nih.gov/books/NBK551517/
Mechanisms of Action and Clinical Applications of Macrolides, https://pubmed.ncbi.nlm.nih.gov/20610825/
Fluoroquinolones: Action and Resistance, https://pubmed.ncbi.nlm.nih.gov/12570763/
Aminoglycosides: Perspectives on Mechanisms of Action and Resistance and Strategies to Counter Resistance, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC90188/
The Prevalence of Antibiotic Prescription
Let's delve into the intricacies of antibiotic prescription, a practice that has seen some intriguing global variations and a worrying trend toward overuse. By shining a spotlight on these issues, we can all better understand the landscape of antibiotic use and what it means for us as a society.
Unraveling the Antibiotic Paradox: A Snapshot of Global Prescription Trends and Their Implications
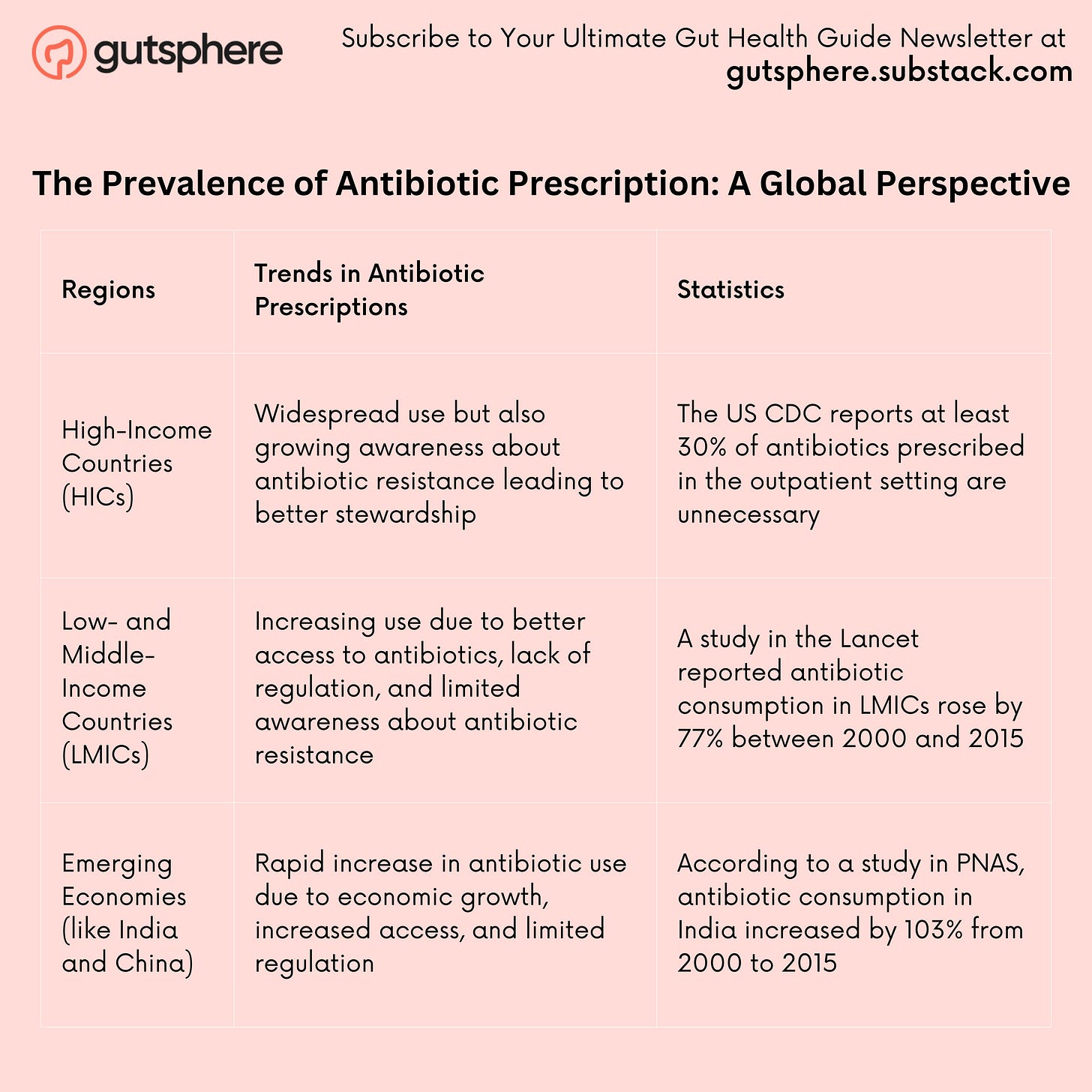
The Prevalence of Antibiotic Prescription: A Global Perspective
Every year, around the world, millions of antibiotics prescriptions are issued. From treating bacterial infections to being wrongly prescribed for viral infections, these small pills have become a significant part of our healthcare practices. In fact, a study by Klein et al. published in 2018 in the journal Proceedings of the National Academy of Sciences revealed that 65% of the population in 76 countries received an antibiotic prescription in 2015 alone[1].
This widespread use of antibiotics is a double-edged sword. On the one hand, antibiotics have saved countless lives and continue to be a cornerstone of modern medicine. On the other hand, their overuse poses a threat that could undermine the very benefits they provide.
Let's look at antibiotic prescription trends across different parts of the globe. In a comprehensive study published in The Lancet Infectious Diseases, Van Boeckel et al. found that from 2000 to 2015, global antibiotic consumption, as measured in defined daily doses (DDD), increased by 65%[2].
Interestingly, the study found that the surge in antibiotic consumption was most pronounced in low and middle-income countries (LMICs). For instance, in India, antibiotic consumption increased by 103%, in China by 79%, and in Pakistan by 65%. In contrast, high-income countries, like the United States and most of Western Europe, saw a modest increase or even a decrease in antibiotic consumption over the same period[3].
Why Are Antibiotics Over-Prescribed?
The overuse of antibiotics can be attributed to several factors. One of the primary ones is the misconception about the role and effectiveness of antibiotics. It's not uncommon for patients to expect antibiotics for conditions where they are not needed, like viral infections, and for healthcare providers to prescribe them just in case[3].
Additionally, in many LMICs, antibiotics can be purchased over the counter without a prescription. This easy availability, coupled with lack of public awareness about appropriate antibiotic use, often leads to unnecessary and incorrect usage[4].
The Implications of Antibiotic Over-Prescription
Overuse and misuse of antibiotics have consequences, and one of the most significant is the rise of antibiotic-resistant bacteria. This phenomenon, known as antibiotic resistance, is when bacteria mutate and become immune to the antibiotics designed to kill them.
The World Health Organization (WHO) considers antibiotic resistance one of the biggest threats to global health today[5]. Every year, in the United States alone, at least 2.8 million people get an antibiotic-resistant infection, and more than 35,000 people die[6].
In the face of this growing crisis, it's clear that we must be more judicious with our use of antibiotics. We need to ensure they are prescribed and used correctly — only when necessary and with the right dose, at the right time, and for the right duration. It's a challenge that will require cooperation and action from all of us: health professionals, policymakers, and, importantly, ourselves as patients.
In the next chapter, we'll explore in more depth the implications of antibiotic resistance and what measures we can take to combat it. So stay with us on this journey as we navigate this complex, invisible battlefield.
Sources and References:
Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. https://www.pnas.org/doi/10.1073/pnas.1717295115 .
Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. The Lancet Infectious Diseases. 2014 Aug 1;14(8):742–50. Link
Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA. 2016 May 3;315(17):1864–73. Link
Non-prescription antimicrobial use worldwide: a systematic review. The Lancet Infectious Diseases. 2011 Sep 1;11(9):692–701. Link
World Health Organization. Antibiotic resistance. Link
Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance (AR/AMR). Link
Antibiotics and the Gut Microbiome
When we discuss the consequences of antibiotic use, it's vital to bring up the impact on our gut microbiome. The gut microbiome, a community of trillions of bacteria residing in our intestines, plays a key role in our health. It helps us digest food, regulates our immune system, and protects against harmful bacteria.
However, when we take antibiotics to kill harmful bacteria, they can also wipe out many beneficial bacteria in our gut. In other words, antibiotics do not discriminate between the 'bad' bacteria causing the infection and the 'good' bacteria necessary for our health.
This disruption to the gut microbiome, known as dysbiosis, can have far-reaching effects on our health. Numerous studies have linked dysbiosis to conditions ranging from obesity and inflammatory bowel disease to mental health disorders like depression and anxiety[1,2].
Furthermore, a study published in the journal mBio found that antibiotics could disrupt the gut microbiome and affect the recovery of the microbial community even after a year of the treatment[3]. This evidence supports the idea that the impact of antibiotics on our gut microbiome is not transient but may have lasting effects.
The recognition of this disruption has led to the emergence of new fields like microbiome-restorative therapies. These therapies, including fecal microbiota transplantation (FMT), aim to restore the gut microbiome after it has been disturbed, for instance, by antibiotics[4].
As we continue our exploration of antibiotics, it's crucial to keep the implications for the gut microbiome in mind. Striking a balance between the life-saving benefits of antibiotics and their potential impact on our gut microbiome represents one of the key challenges in modern medicine.
In the next part, we'll delve into the fascinating world of the gut microbiome in more detail. So stay with us as we navigate this vast, invisible ecosystem inside us.
Sources and References:
Dysbiosis of the gut microbiota in disease. Microbial ecology in health and disease. 2015 Dec;26(1):26191. Link
Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress. 2017 Mar 1;7:124-36. Link
Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome versus Long-Term Microbial Shifts in Feces. mBio. 2015 Dec 8;6(6):e01693-15. Link
European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017 Apr 1;66(4):569-80. Link
Wrapping Up Part One: Unmasking Antibiotics and Their Global Usage
In this first part of our exploration, we've journeyed through the world of antibiotics, demystifying their mechanisms, and understanding the various classes at our disposal. We've seen how these powerful allies in our fight against bacterial infections operate, the benefits they bring, and the potential drawbacks they carry, especially concerning our gut microbiome.
We also took a panoramic view of the global landscape of antibiotic usage, noting the prevalence of antibiotic prescriptions. We learned that, while antibiotics have been instrumental in combating numerous bacterial diseases, their impact isn't only immediate. The consequences extend beyond our individual bodies and span across generations.
As we conclude this part, we're left with several questions. How do our decisions about antibiotic usage today affect future generations? What role does childhood antibiotic use play in a person's life, and how do we balance the risks and benefits?
Our voyage into the world of antibiotics is far from over. As we set our compass towards the next chapter of our exploration, we're about to delve into uncharted territory.
Next:
In Part Two, we'll look at the ripple effect of antibiotics across generations, touching upon childhood antibiotic use and the delicate task of balancing its risks and benefits. We'll also look at the intergenerational impact of antibiotics, an emerging and essential field of research that has been capturing significant public interest.
Stay with us as we prepare to set sail into these fascinating waters. The journey continues...
Our sincere request to you is to share the newsletter with your friends, family, and community so that they can benefit from the content. Also it will help us grow the newsletter, and eventually, as we release more content, digital tools, and more we will enable people around the world to live chronic disease free.
Subscribe
If you haven’t already subscribed then our sincere request, please subscribe.
Feedback
Also, please give us feedback so that we can improve the content. And if there are any topics that you want us to cover please send us your questions and topics. Furthermore, if you try any of the things we provided information please share your experience with us.
Thank You
Gutsphere Team
Disclaimer
Please note that the information provided in this newsletter is for informational purposes only and should not be considered as a substitute for professional medical advice, diagnosis, or treatment. If you have any concerns or questions about our health, please consult with a licensed healthcare professional. The information contained in this newsletter is not intended to diagnose, treat, cure, or prevent any disease. The publisher and authors of this newsletter assume no responsibility for any adverse effects that may result from the use of the information contained herein.