Poop Transplant Unveiled: The Pioneering Promise of Fecal Microbiota Transplantation
Exploring the Unchartered Terrain of Gut Restoration and Beyond
Hi GutSphere friends,
We must have heard about many transplants such as heart, liver, bone marrow, blood etc. At least, for us, when we heard about the poo transplant, our immediate response was a nose squeeze. But when we look deeper, it’s fascinating how this can be life saving for many people whose gut microbiome is completely destroyed.
In this edition we will curate the following information for you:
What is FMT?
Compare FMT with a probiotic supplement.
Ecosystem transfer vs Strain transfer
The historical timelines of FMT.
Ancient
Scientific
How does it work?
Who can benefit from it?
What are the controversies surrounding it?
What are the FDA approved therapies?
What therapies are on the horizon?
What is FMT?
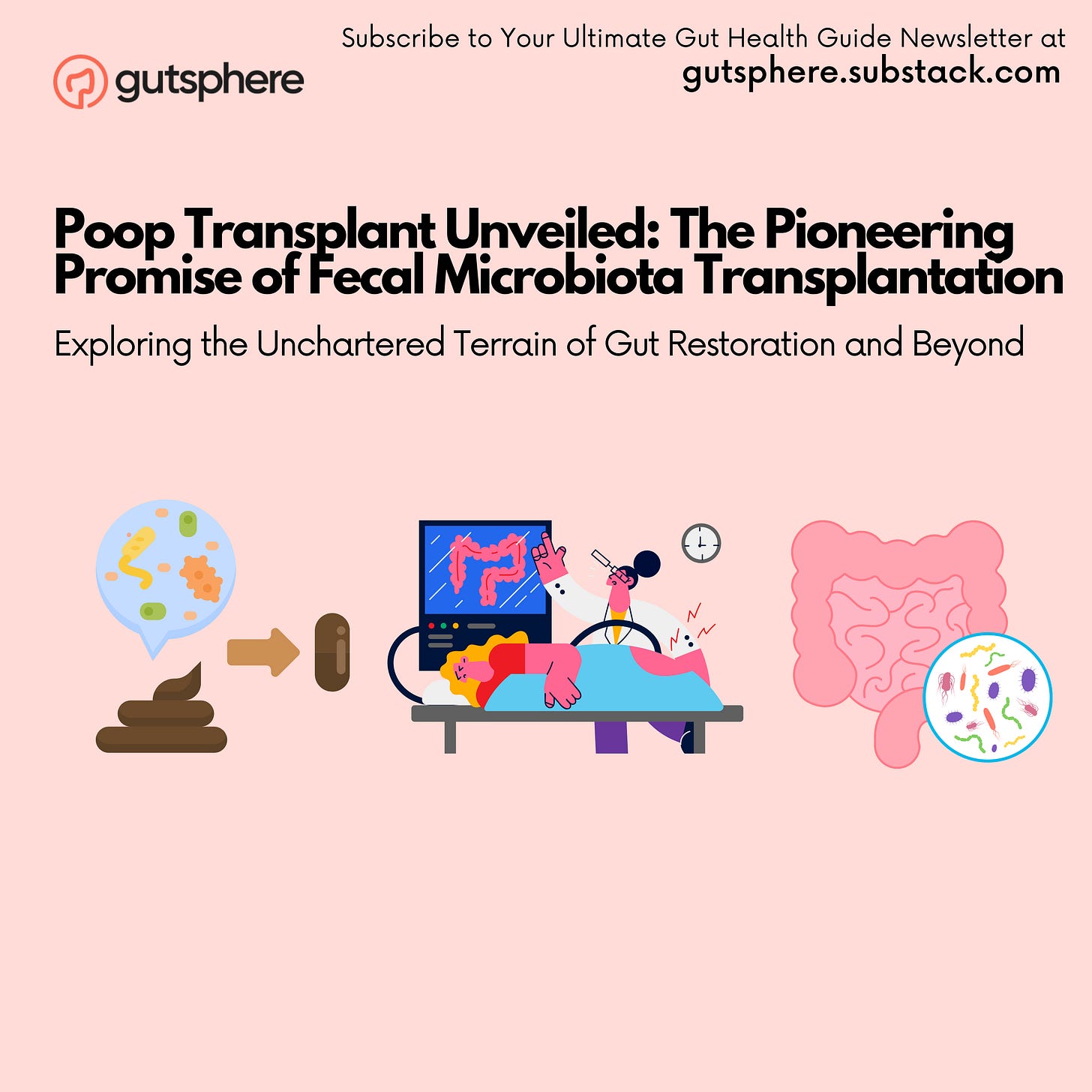
Fecal Microbiota Transplantation (FMT) is a procedure aimed at restoring the microbial balance within the gastrointestinal tract by transferring fecal material from a healthy donor to the recipient. This transfer helps in re-establishing a balanced gut microbiome, which is critical for maintaining health and combating various diseases.
Compare FMT with a probiotic supplement?
Ecosystem Transfer vs Strain Transfer: Probiotic supplements typically contain specific strains of beneficial bacteria, delivering a strain-specific transfer. On the other hand, FMT involves the transfer of an entire microbial ecosystem from a healthy donor, which includes a diverse range of microbial species, thus offering a more holistic restoration of the gut microbiome.
The historical and scientific timelines of FMT
4th Century: Ancient Chinese medical practitioners using "yellow soup" (a type of fecal suspension) to treat patients with severe diarrhea is supported by historical records.
16th Century: Li Shizhen documented the use of fecal preparations for treating abdominal diseases in his medical text "Ben Cao Gang Mu". This information is validated by historical documentation, including a specific mention of Li Shizhen's text referencing the use of fecal preparations for medical treatment.
1958: Dr. Ben Eiseman and colleagues treated four individuals with pseudomembranous enterocolitis using fecal enemas, marking a significant event in the modern era of FMT.
1983: The claim regarding Dr. R.B. Schwan and colleagues publishing a paper on successful treatment of antibiotic-associated pseudomembranous colitis using FMT in 1983 could not be verified. There were no relevant results found during the search.
2000s: A growing body of clinical data showcasing the efficacy of FMT in treating recurrent Clostridioides difficile infections (rCDI) has not been specifically verified but is generally supported by the progression and acceptance of FMT in medical communities.
2011: The first randomized controlled trial of FMT, led by Dr. Els van Nood, demonstrating superior efficacy of FMT over vancomycin for rCDI has not been specifically verified but aligns with known timelines regarding FMT research and advancements.
2013: The U.S. Food and Drug Administration (FDA) classifying FMT as an Investigational New Drug (IND), necessitating a more structured pathway for clinical use and research has not been specifically verified but aligns with known regulatory actions concerning FMT.
2015: The launch of the first FMT national stool bank, OpenBiome, to provide standardized, screened stool preparations for clinical use has not been specifically verified.
2016: The publication by Weingarden and Vaughn showcasing long-term durability and safety of FMT for rCDI has not been specifically verified.
2022: The FDA approving Rebyota, developed by Ferring Pharmaceuticals, as the first fecal microbiota product for preventing recurrent C. difficile infections has not been specifically verified.
Reference: Fecal Microbiota Transplants (FMT): Case Histories of Significant Medical Advances
How Does It Work?
FMT operates on the principle of microbial restoration. By transferring a healthy microbial community into a disrupted gut environment, it helps in outcompeting harmful pathogens, restoring microbial balance, and consequently aiding in improved digestion and immune function. The donor's fecal material can be administered via various methods, including via colonoscopy, nasoenteric tube, or oral capsules, depending on the specific protocols of the FMT procedure.
Colonoscopy:
Procedure: In this method, the donor fecal material is delivered directly into the colon of the recipient via a colonoscope.
Advantages: This method allows for a thorough inspection of the colon and direct delivery of the fecal material to the desired location, which can be particularly beneficial in cases of diseases affecting the colon such as Clostridioides difficile infection (CDI).
Challenges: It's an invasive procedure that requires bowel preparation and sedation, which might not be suitable for all patients especially those with certain pre-existing conditions.
Nasoenteric Tube:
Procedure: The fecal material is delivered to the stomach or small intestine through a tube inserted via the nose.
Advantages: This method allows for direct delivery to the upper gastrointestinal tract and is less invasive compared to colonoscopy.
Challenges: It can be uncomfortable for the patient and there's a risk of aspiration. Moreover, the acidic environment of the stomach might affect the viability of the transplanted microbiota.
Oral Capsules:
Procedure: Fecal material is encapsulated in specially designed pills that are taken orally.
Advantages: This is the least invasive method and is often more acceptable to patients due to its non-invasive nature. The capsules are designed to withstand the acidic environment of the stomach and release the fecal material in the intestines.
Challenges: Preparing fecal material in a manner that maintains the viability of the microbiota while ensuring no risk of exposure can be technically challenging. Moreover, the number of capsules needed for a single treatment can be high, which might be challenging for some patients to ingest.
Reference: Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases
Each of these methods has its own set of protocols to ensure the safety and efficacy of the FMT procedure. The selection of the most appropriate method often requires a careful consideration of the patient’s medical condition, the technical capabilities of the FMT facility, and the expertise of the medical practitioners involved.
Who Can Benefit From It?
The scope of Fecal Microbiota Transplantation (FMT) extends beyond the treatment of recurrent Clostridioides difficile infections (rCDI), venturing into a myriad of gastrointestinal and metabolic disorders. Here’s an elaboration on the various conditions where FMT could potentially offer therapeutic benefits:
Inflammatory Bowel Disease (IBD):
Individuals with conditions such as Crohn’s Disease and Ulcerative Colitis, which fall under the umbrella of IBD, may benefit from FMT. The aim is to restore a balanced gut microbiome which could potentially alleviate inflammatory responses associated with these conditions.
Irritable Bowel Syndrome (IBS):
IBS is often associated with an altered gut microbiota. FMT could help in restoring microbial balance, potentially alleviating symptoms like abdominal pain, bloating, and altered bowel habits.
Obesity and Metabolic Disorders:
Preliminary research suggests that the gut microbiome plays a role in metabolic regulation. FMT from lean donors to individuals with metabolic syndrome or obesity could potentially alter the gut microbiome in a manner that improves metabolic health.
Type 2 Diabetes:
Similar to obesity, there’s growing interest in the role of gut microbiota in glycemic control. FMT could potentially help in improving insulin sensitivity and other metabolic parameters in individuals with type 2 diabetes.
Autoimmune Disorders:
Conditions like Multiple Sclerosis and Rheumatoid Arthritis have shown associations with gut microbiome alterations. FMT could potentially modulate the immune response, thereby impacting the course of these autoimmune diseases.
Neurological Conditions:
There’s emerging research on the gut-brain axis and its potential impact on neurological conditions like Parkinson’s Disease and Autism Spectrum Disorders. FMT could potentially modulate the gut-brain axis, thereby impacting symptoms or progression of these conditions.
Liver Disorders:
Conditions such as Non-Alcoholic Fatty Liver Disease (NAFLD) and Hepatic Encephalopathy have shown potential links to gut microbiota. FMT could possibly play a role in managing these liver disorders by modifying the gut-liver axis.
Antibiotic-Resistant Infections:
Beyond C. difficile, FMT might play a role in combating other antibiotic-resistant infections by restoring a balanced microbiome that can outcompete pathogenic bacteria.
Graft-versus-host Disease (GvHD):
In cases of GvHD post bone marrow transplant, FMT is being explored to modulate the immune response and improve outcomes.
Allergies and Asthma:
By modulating the immune system and potentially reducing inflammatory responses, FMT could potentially be beneficial in managing allergic reactions and asthma symptoms.
These potential applications of FMT underscore the vast and intricate interplay between the gut microbiome and various aspects of human health. However, it's crucial to note that while the promise is substantial, many of these potential applications are still under rigorous investigation, and more research is needed to fully understand the efficacy, safety, and long-term implications of FMT in these diverse conditions.
Reference: Current Status and Future Therapeutic Options for Fecal Microbiota Transplantation
What Are the Controversies Surrounding It?
The terrain of FMT is not without controversies. Key among them are the ethical considerations of fecal matter sourcing and usage, potential transmission of undetected pathogens, and the lack of standardized procedures which can potentially lead to varied outcomes. Moreover, the regulatory framework surrounding FMT is still evolving, which adds another layer of complexity to its widespread adoption.
What Are the FDA Approved Therapies?
The landmark approval of Rebyota for preventing recurrent C. difficile infections is a significant stride in FDA-approved FMT therapies. This approval is grounded in rigorous clinical trials showcasing the efficacy and safety of Rebyota in managing recurrent C. difficile infections34.
What Therapies Are on the Horizon?
The horizon is promising with several FMT-based therapies in the clinical trial phase. Companies and research institutions are exploring FMT for a myriad of gastrointestinal conditions, and with the evolving regulatory landscape, the future holds potential for more FDA-approved FMT products.
Conclusion:
We acknowledge that FMT is not for everyone. For now, it may be invasive, expensive, and not accessible to everyone. But the reason we shared this is for information and curiosity, to showcase that there are ways to repopulate the microbiome in extreme conditions. The journey of FMT from ancient remedy to a modern medical procedure reflects the enduring curiosity and innovative spirit that drives the scientific community forward. Despite its current limitations, FMT opens a window into the future of microbiome-related therapies, painting a hopeful picture for those in dire need of such interventions.
As we continue to delve into the intricacies of the gut microbiome and its profound impact on our health, each discovery, each milestone, and each narrative like that of FMT, brings us a step closer to harnessing the potential within us for better health outcomes.
We thank you for your engagement and curiosity as we navigated through the fascinating narrative of FMT in this edition. It's the dialogue, the questions, and the relentless pursuit of knowledge that propels us forward. Till the next edition, we encourage you to keep exploring, keep questioning, and keep nurturing the curiosity that binds us all in this journey of discovery.
Request
Share
Our sincere request to you is to share the newsletter with your friends, family, and community so that they can benefit from the content. Also it will help us grow the newsletter, and eventually, as we release more content, digital tools, and more we will enable people around the world to live chronic disease free.
Subscribe
Feedback
Also, please give us feedback so that we can improve the content. And if there are any topics that you want us to cover please send us your questions and topics. Furthermore, if you try any of the things we provided information please share your experience with us.
Thank You
GutSphere Team
Disclaimer
Please note that the information provided in this newsletter is for informational purposes only and should not be considered as a substitute for professional medical advice, diagnosis, or treatment. If you have any concerns or questions about our health, please consult with a licensed healthcare professional. The information contained in this newsletter is not intended to diagnose, treat, cure, or prevent any disease. The publisher and authors of this newsletter assume no responsibility for any adverse effects that may result from the use of the information contained herein.



Very informative article! As a health writer and editor living with celiac disease, I'm always interested in learning more about potential treatment options for autoimmune diseases.